Few subjects have captured the attention of boiler cycle chemistry experts and research dollars in the past 10+ years as flow accelerated corrosion (FAC). Having previously been most concerned about failures occurring in higher pressure areas of the cycle, FAC is now a primary concern as over 60% of all utilities have reported finding it in their facilities. Defined as localized rapid metal loss resulting in tube wall thinning of carbon and low alloy piping, FAC is known to occur in the mid-temperature regions of the cycle, between 250F and 400F. In high pressure boiler cycles, these temperatures exist in boiler feedwater or “LP” (low pressure) and “IP” (intermediate pressure) sections of a Heat Recovery Steam Generator (HRSG).
Aside from the obvious concern of catastrophic failure of feedwater piping, FAC is a significant safety concern. Unlike high pressure boiler tube failures that occur inside a structure, FAC occurs in areas of the plant that are in close proximity to plant personnel. The first documented failure was in December 1986 at a nuclear power plant in Virginia. Termed “erosion-corrosion,” the failure resulted in four fatalities. The Electric Power Research Institute (EPRI) and Occupational Safety and Health Administration (OSHA) both became involved in documenting FAC cases and identifying the root causes. In 1996 EPRI published the results of their investigation on industry experiences. That same year, OSHA issued a hazards bulletin on FAC. The culmination of these efforts led to the publication of guidelines to minimize the risk of FAC in 2004. These guidelines, along with increased vigilance of inspections and attention to cycles chemistry in the industry, have led to a reduction in FAC instances.
 FAC metal loss is best described as the absence of the protective iron oxide layer that, when present, limits corrosion in boiler systems. Without this protection, the surface is free to react with the passing water (or steam-air mixture called two-phase FAC) and metal loss is rapid. Classic FAC attack is bare (shiny) metal with a scalloped appearance in the direction of flow.
FAC metal loss is best described as the absence of the protective iron oxide layer that, when present, limits corrosion in boiler systems. Without this protection, the surface is free to react with the passing water (or steam-air mixture called two-phase FAC) and metal loss is rapid. Classic FAC attack is bare (shiny) metal with a scalloped appearance in the direction of flow.
Factors that affect FAC are: flow velocity, geometry, metallurgy, temperature, dissolved oxygen concentration, and pH. Piping geometry and flow velocity go hand in hand, as failures typically occur in areas of higher velocity relative to adjacent areas like the outside of tube bends or where a tube intersects a header, so periodic inspection of these areas in your boiler cycle is highly recommended. There are many boiler service companies that will perform these inspections and offer retrofit solutions through a change in piping geometry and/or a change of metallurgy in sensitive areas to higher chrome (>0.1%) alloys. To understand why iron oxides would not develop in these areas, researchers focused on the sometimes complex physics and chemistry of iron oxides. Iron exists in two oxidation states (Fe+2 and Fe+3) and a variety of structures, so there are sixteen known iron oxides and oxyhydroxides (Cornell and Schwertmann, 2003), each with different properties. Magnetite (Fe3O4) is formed in a boiler circuit under anaerobic conditions and is very stable, dense and uniform at high temperatures (>400F). Between 250F and 400F, magnetite forms less rapidly and is porous and irregular; and below 250F, magnetite forms very slowly.
Therefore, in the temperature range that FAC is of concern, magnetite is of less help when it comes to corrosion protection than at the higher temperatures. EPRI research has shown that alpha structured ferric oxide, known as hematite (α-Fe2O3) can strengthen the porous magnetite layer and result in a more stable protective layer. Hematite is formed in oxidizing environments, so having some dissolved oxygen present in feedwater circuits (<10 ppb) is now seen as beneficial. This has led many plants to abandon the use of chemical oxygen scavengers in all ferrous systems where continuously well operated mechanical deaeration and minimal air in-leakage is the norm. Iron oxide solubility is also very dependent upon pH especially in the typical FAC temperature range as pictured below.
To aid in the formation of iron oxide formation, the historical feedwater pH control ranges of 8.8 – 9.2 have been elevated to 9.4 – 9.8 for all-ferrous systems and 9.2 – 9.6 for mixed m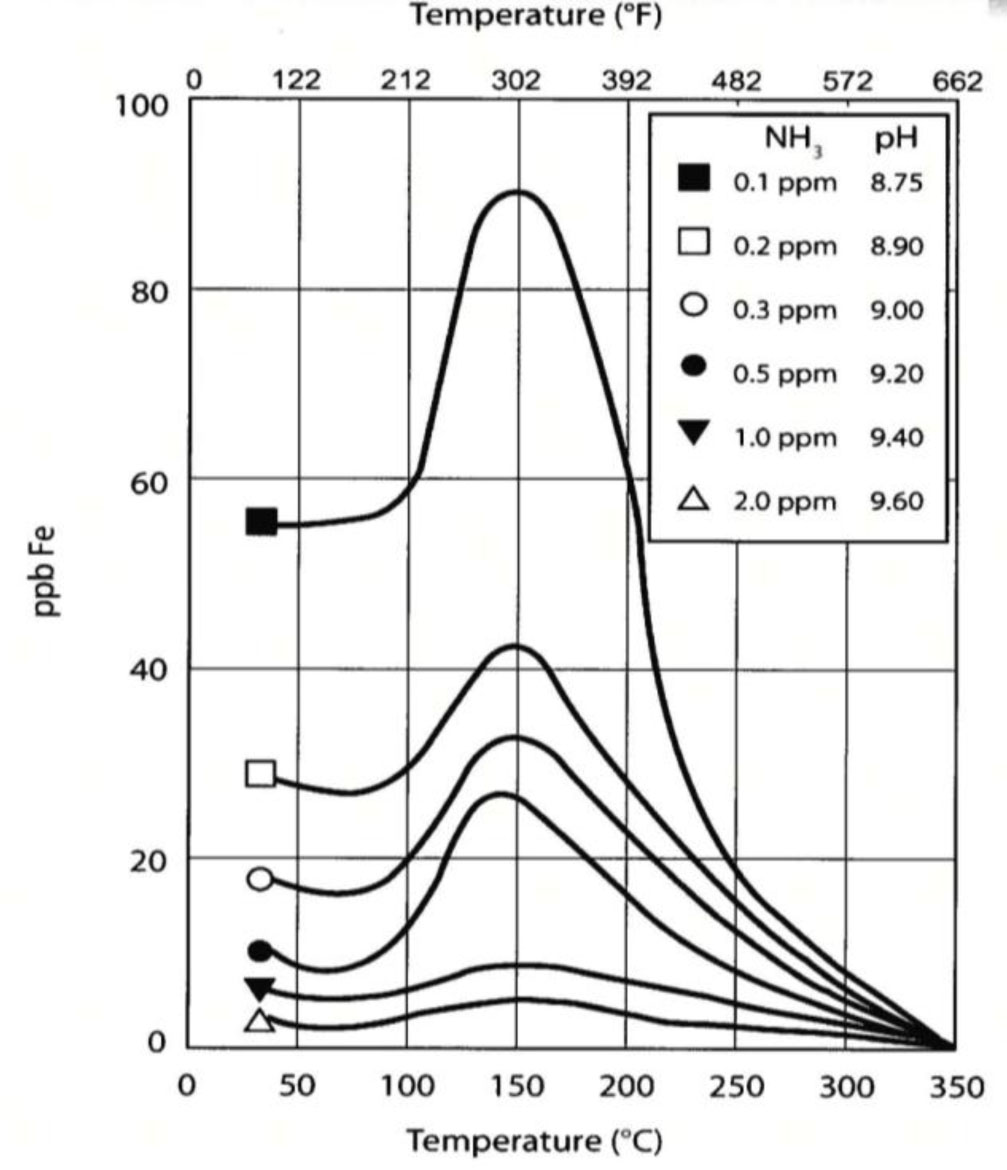 etallurgy (copper-based feedwater heater component) systems.
etallurgy (copper-based feedwater heater component) systems.
Total iron levels in the feedwater is the best metric for FAC while the plant is on-line. Effective feedwater chemistry will yield total iron levels of less than 10 ppb. Since each plant is unique with respect to operating regimes, system metallurgy, and environmental or steam host restrictions, we recommend contacting your U.S. Water representative for specific recommendations regarding your plant with respect to FAC prevention.
